We are currently focusing on two topics: the blood-brain barrier (BBB) and neurovascular coupling.
Neurons in the brain are extremely sensitive to their extracellular chemical environment and highly metabolic. Despite representing only 2% of the body weight, our brain consumes 20% of our body’s energy at rest and has limited ability to store energy. To meet these unique challenges, brain vasculature has two distinct physiological features not present in the peripheral vasculature:
- The blood-brain barrier (BBB) provides a protected and homeostatic brain environment. The BBB is formed by a single layer of Endothelial cells that lines the walls of brain blood vessels and serves as a physiological barrier between the blood and neural tissue.
- The dynamic regulation of blood flow meets moment-to-moment changes in regional brain energy demand. Neural activity rapidly increases regional blood flow, within hundreds of milliseconds, a process called neurovascular coupling.
Traditionally, endothelial cells forming the blood vessel walls in the brain were thought to play a passive role in supplying the brain with oxygen and nutrients. Our work so far has changed this view by revealing the specialization and active functions of brain endothelial cells necessary to regulate brain physiology.
TOPIC 1: The blood-brain barrier (BBB)
Recent progress:
Historically, the restricted permeability of brain vasculature has been attributed to tight junctions, specialized contacts between adjacent Endothelial cells that prohibit paracellular passage of water-soluble molecules. However, substances can also cross Endothelial cells by transcytosis, a process by which material enters endocytic vesicles that traffic across the cell and release their contents on the other side. We discovered that transcytosis is actively inhibited in CNS Endothelial cells. Moreover, we identified a molecular pathway that is necessary for the suppression of transcytosis in CNS Endothelial cells. We showed that inhibiting this pathway causes the BBB to become permeable. Thus, tight junctions alone are not sufficient for BBB integrity. We also discovered that the rate of transcytosis is dynamically modulated during development and disease. Our findings imply that the molecular pathways inhibiting transcytosis could be targeted to open the BBB and deliver drugs to the CNS.
Our central hypothesis is that brain Endothelial cells express a special set of genes that govern formation and maintenance of the BBB. We originally identified over 200 genes highly expressed in cortex Endothelial cells but with low or undetectable expression in lung Endothelial cells. We then showed that one of these genes, Mfsd2a, functions as a transcytosis inhibitor to regulate the BBB. Mice and zebrafish lacking Mfsd2a have a leaky BBB, resulting from up-regulation of transcytosis without disruption of tight junctions. Recently, we established Mfsd2a’s mechanism of action: lipids translocated by Mfsd2a establish a unique lipid composition in the CNS endothelial cell plasma membrane that inhibits caveolae vesicle formation, thereby suppressing transcytosis. In unpublished work, we also found that Mfsd2a is down-regulated after stroke and in aging when the BBB is compromised. Our Mfsd2a studies serve as a proof of principle, both conceptually and technically, for assessing the functions of other BBB-enriched genes identified in our screen.
Current questions:
- What endothelial cell molecular specializations promote BBB integrity?
- BBB properties are not intrinsic to Endothelial cells but require active maintenance from the brain. How do astrocytes and pericytes maintain the BBB?
- Peripheral inflammation profoundly influences brain health. How do brain Endothelial cells transmit peripheral immune signals to the brain?
TOPIC 2: Dynamic regulation of blood flow (neurovascular coupling)
Recent progress:
The conventional view holds that neurons and astrocytes release signals that act directly on smooth muscle cells, resulting in vasodilation and increase in blood flow. Endothelial cells are thought to play a passive role in this process. Contrary to this view, we recently discovered that arteriolar Endothelial cells actively relay signals from neurons to smooth muscle cells.
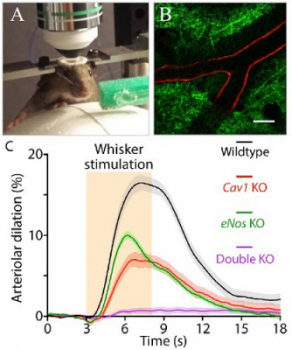
We recently identified ultrastructural specializations in arteriolar Endothelial cells essential for neurovascular coupling. Specifically, we discovered that – unlike capillary Endothelial cells that express Mfsd2a and thus have few caveolae – arteriolar Endothelial cells do not express Mfsd2a and contain abundant caveolae. Using two-photon microscopy to image neural activity and vascular dynamics simultaneously in the barrel cortex of awake mice during whisker stimulation (Fig.1A-B), we showed that neurovascular coupling is impaired by acute genetic perturbations that eliminate caveolae in arteriolar Endothelial cells, but not in neighboring smooth muscle cells. We further demonstrated that this novel caveolae-mediated pathway operates independently of the well-known nitric oxide pathway: simultaneous ablation of both pathways is necessary to abolish neurovascular coupling (Fig.1C).
Current questions:
- 2a. How are the neural signals propagated through Endothelial cells and to smooth muscle cells?
- 2b. What restricts neurovascular coupling to a specific region?
- 2c. What are the consequences of impaired neurovascular coupling for brain function and health?